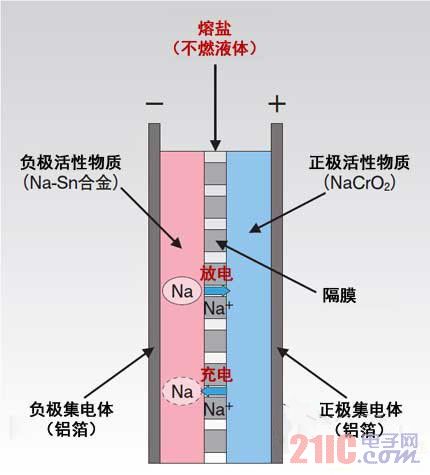
A representative product of a high output power high energy density rechargeable battery is now a lithium (Li) ion battery. Lithium-ion batteries have been widely adopted from small electronic devices such as mobile phones to large-scale products such as hybrid electric vehicles (HEVs) and electric vehicles (EVs). Not only that, lithium-ion batteries are also used to store electricity from natural energy sources such as solar power and wind power, as well as backup power sources for office buildings and factories. This article refers to the address: http:// However, lithium-ion batteries use a flammable electrolyte, so there is a risk of thermal runaway due to high temperatures. From a resource point of view, the distribution of lithium is more in countries and regions such as South America, China and Australia, so it is impossible to eliminate concerns about stable procurement. Incombustible molten salt as electrolyte We need to produce a high-density rechargeable battery without worrying about causing thermal runaway, stable supply of resources, and high energy density. The research and development team of Sumitomo Electric Industries and Kyoto University's School of Energy Sciences, Ph.D. and others, is developing a new type of battery for molten salt electrolyte batteries (Fig. 1). Figure 1: The battery capacity of the molten salt electrolyte battery co-produced by Sumitomo Electric Industries and Kyoto University Sugawara et al. is 9 kWh per section. This was stacked in four sections to form a 36 kWh battery pack. This is a rechargeable battery that is charged and discharged by moving sodium (Na) ions (Na+) between the positive electrode and the negative electrode. The electrolyte is sodium bis(fluorosulfonyl)imide (NaFSA) and bis(fluorosulfonyl)imide. Potassium (KFSA), two non-flammable molten salts. According to reports, even if air is mixed from the outside due to shocks such as earthquakes and accidents, it will not ignite, and there will be no thermal runaway due to excessive charging and increased battery temperature. For the constituent members other than the electrolytic solution, the negative electrode active material is a sodium tin (Na-Sn) alloy, and the positive electrode active material is sodium chromite (NaCrO 2 ), which is sandwiched between the two active materials (previously impregnated as mentioned above) The molten salt is made of glass fiber, and the current collector is made of aluminum foil (Fig. 2)*1. These materials are non-combustible materials and are “made of resource-rich raw materials†(Tian Bianjing Yilang, director of the new business development department of Sumitomo Electric Business Planning Department). As an important material, Na is abundant in seawater and is rich in resources. Figure 2: Cell structure of the new battery The electrolyte is made of a non-flammable molten salt or the like, and since it is entirely composed of a non-combustible material, there is no need to worry about fire or thermal runaway. It is said that all resources are abundant. The Na-Sn alloy is a sodium tin alloy and NaCrO2 is a sodium chromite. 1: The Na-Sn alloy is different from the metal Na, and it remains stable even when it is exposed to water in the atmosphere, and there is no problem in safety. Chromium in NaCrO2 exists in the form of trivalent, and does not form hexavalent even when charged and discharged. Reduce operating temperature by mixing molten salt Sumitomo Electric and the R&D team at Kyoto University pay attention to Na not only because of its abundant reserves. Also, the energy density of the battery cells based on Na ion conduction is high. But there is a problem here. Although batteries that use Na-ion conduction like sodium sulfide (NaS) batteries have been used before, they cannot be used at operating temperatures below 100 °C. For example, the operating temperature of the NaS battery is set to 300 to 350 °C. If the working temperature is high, a large amount of energy is required to reach the temperature, and all-round management such as prevention of burns and burns is indispensable. Conversely, without the presence of professional managers, NaS batteries are difficult to use. The R&D team has prototyped a new type of battery, but needs a salt that allows Na ions to conduct at low temperatures. Therefore, the R&D team developed the aforementioned molten salt. The melting point of these molten salts is 57 ° C. If it is only discharged, the Na ions are conducted at a temperature of 57 ° C or higher. If charging is included, the Na ions can be conducted at a temperature of 80 ° C or higher. According to Inazawa Shinji, head of the electrochemistry team of the Institute of Metal Inorganic Materials Technology, Sumitomo Electric and Electronic Materials Research Institute, the key is to form a mixture of NaFSA and KFSA. In fact, the melting points of NaFSA and KFSA were as high as 106 ° C and 96 ° C, respectively, when used alone. When you mix the two, the melting point will drop (Figure 3). Inazawa Shinji said, "The size of Na and K ions is different. After mixing substances with different ionic radii, you can get the effect of lowering the melting point." By melting point reduction, Na ions can also conduct at temperatures below normal. Figure 3: Newly developed molten salt The melting point is reduced by mixing two non-flammable salts of sodium bis(fluorosulfonyl)imide (NaFSA) and potassium bis(fluorosulfonyl)imide (KFSA). Helps reduce battery pack size The prototype battery has an operating temperature range of 57 to 190 °C. However, according to Inazawa, it usually works at 80 to 90 ° C (optimal temperature is 90 ° C). At this temperature, NaFSA and KFSA melt, flow smoothly like water, and separate into Na ions, K ions, and FSA ions. At 57 ° C, it is more viscous, so the Na ions are difficult to move. During charging, Na ions are eluted from the positive NaCrO2 and moved to the negative electrode. On the contrary, Na ions are eluted from the Na-Sn alloy of the negative electrode and are transferred to the positive electrode. Since Na ions exist alone and can move between the electrodes, there is no need to dissolve the solvent of Na ions. On the other hand, in a lithium ion battery, since lithium ions are in close contact with diethyl carbonate (DEC) as a solvent to form a so-called solvent to move in the electrolyte, a solvent is required. Sumitomo Electric made a 36kWh battery pack with a capacity of 9kW per section and consisting of four sections, and set up a field test at the company's Osaka Manufacturing Plant. Various characteristics of the battery were investigated by overcharging or the like. The volume when the new battery is made into a battery pack is about half of the capacity (36 kWh) lithium-ion battery pack and about 1/4 of the capacity of the NAS battery pack, according to estimates by Sumitomo Electric. Unlike the battery pack of a lithium-ion battery, the new battery requires no heat dissipation space, can be arranged at a high density, and requires only a simple cooling device, so that it can be miniaturized. The cooling device is an emergency countermeasure against the fire in the vicinity of the battery pack due to a fire or the like. Since the molten salt decomposes and generates gas at a temperature exceeding 190 ° C, the cooling device is installed to prevent this. The battery itself has an energy density of up to 290Wh/L. The practical target period for new batteries is 2015. In the future, Sumitomo Electric plans to use it for power storage applications such as medium-sized power grids and homes, as well as a wide range of applications, including in-vehicle applications such as trucks and buses. It will be able to look for and lower the battery while repeatedly evaluating and improving the battery. Molten salt*2 working at temperature. Establishing production technology and focusing on standardization and improvement of structure will be the subject of future research. New batteries cannot fully replace lithium-ion batteries. However, after the battery is put into practical use, the resources required for the battery can be dispersed and the safety of the battery can be improved. This is a technology that helps build a sustainable society. 0-10V Dimmable LED Driver,use 0-10V dimmer,Waterproof IP65 Aluminum housing series, 1-300W CE/ROHS/SAA/ETL/TUV/EMC,Input voltage can be both 110V and 220V,Wattage can be 1-80W, DALI/TRIAC/0-10V/PUSH dimmable and non dimmable, flicker free,noise free,load free, Perfect dimming curve, PF>0.95,constant current 350mA 700mA 900mA 1200mA,constant voltage 12V 24V 36V, parameters can be customization,OEM/ODM is supported,Same appearances but different sizes, forming a perfect product line,Use for led panel light,led strip light and other indoor led lights IP65 0-10v Dimmable Led Driver 0-10V Driver,240W 0-10V Driver,24V Dimmable LED Driver HAURUI LIGHTING CO.,LTD , http://www.huaruileddriver.com

2: Sumitomo Electric is promoting the development of other batteries (redox flow batteries) for high-capacity applications at the MWh level. The molten salt electrolyte battery also has a mass production problem, and thus it is currently difficult to increase the capacity. The redox flow battery is a rechargeable battery that is charged and discharged by a redox reaction of vanadium (V) plasma. It is suitable for irregular and variable charging and discharging, and can accurately monitor and control the amount of stored electricity, so it is suitable for power storage applications for solar power generation and wind power generation.